What Chemicals Help Reduce A Change In Ph When Acids Are Added To A Solution?
pH, Buffers, Acids, and Bases
Acids dissociate into H+ and lower pH, while bases dissociate into OH– and raise pH; buffers can absorb these excess ions to maintain pH.
Learning Objectives
Explicate the composition of buffer solutions and how they maintain a steady pH
Key Takeaways
Key Points
- A basic solution will accept a pH above 7.0, while an acidic solution volition have a pH below 7.0.
- Buffers are solutions that contain a weak acid and its a conjugate base; every bit such, they can absorb excess H+ ions or OH– ions, thereby maintaining an overall steady pH in the solution.
- pH is equal to the negative logarithm of the concentration of H+ ions in solution: pH = – log[H+].
Fundamental Terms
- alkaline: having a pH greater than 7; basic
- acidic: having a pH less than 7
- buffer: a solution composed of a weak acrid and its conjugate base of operations that can be used to stabilize the pH of a solution
Self-Ionization of Water
Hydrogen ions are spontaneously generated in pure water by the dissociation (ionization) of a small percent of water molecules into equal numbers of hydrogen (H+) ions and hydroxide (OH–) ions. The hydroxide ions remain in solution considering of their hydrogen bonds with other water molecules; the hydrogen ions, consisting of naked protons, are immediately attracted to un-ionized water molecules and course hydronium ions (H30+). By convention, scientists refer to hydrogen ions and their concentration as if they were free in this state in liquid water.
[latex]2\text{H}_2\text{O}\leftrightharpoons\text{H}_3\text{O}^{+}+\text{OH}^{-}[/latex]
The concentration of hydrogen ions dissociating from pure h2o is ane × 10-7 moles H+ ions per liter of water. The pH is calculated as the negative of the base 10 logarithm of this concentration:
[latex]\text{pH}=-log[H^{+}][/latex]
The negative log of 1 × x-vii is equal to vii.0, which is also known as neutral pH. Human cells and blood each maintain near-neutral pH.
pH Scale
The pH of a solution indicates its acidity or basicity (alkalinity). The pH scale is an inverse logarithm that ranges from 0 to 14: anything below 7.0 (ranging from 0.0 to half-dozen.9) is acidic, and anything higher up 7.0 (from 7.i to 14.0) is basic (or alkaline metal ). Extremes in pH in either management from 7.0 are usually considered inhospitable to life. The pH in cells (6.8) and the claret (7.4) are both very close to neutral, whereas the environment in the stomach is highly acidic, with a pH of 1 to two.

The pH scale: The pH calibration measures the concentration of hydrogen ions (H+) in a solution.
Non-neutral pH readings result from dissolving acids or bases in water. Using the negative logarithm to generate positive integers, high concentrations of hydrogen ions yield a low pH, and low concentrations a high pH.
An acid is a substance that increases the concentration of hydrogen ions (H+) in a solution, usually by dissociating ane of its hydrogen atoms. A base provides either hydroxide ions (OH–) or other negatively-charged ions that react with hydrogen ions in solution, thereby reducing the concentration of H+ and raising the pH.
Strong Acids and Strong Bases
The stronger the acid, the more readily information technology donates H+. For example, hydrochloric acid (HCl) is highly acidic and completely dissociates into hydrogen and chloride ions, whereas the acids in lycopersicon esculentum juice or vinegar do non completely dissociate and are considered weak acids; conversely, strong bases readily donate OH– and/or react with hydrogen ions. Sodium hydroxide (NaOH) and many household cleaners are highly basic and give up OH– rapidly when placed in h2o; the OH– ions react with H+ in solution, creating new h2o molecules and lowering the amount of free H+ in the organisation, thereby raising the overall pH. An example of a weak basic solution is seawater, which has a pH near viii.0, close enough to neutral that well-adapted marine organisms thrive in this alkaline environment.
Buffers
How tin organisms whose bodies require a near-neutral pH ingest acidic and basic substances (a human drinking orange juice, for example) and survive? Buffers are the primal. Buffers commonly consist of a weak acid and its conjugate base; this enables them to readily blot excess H+ or OH–, keeping the system's pH within a narrow range.
Maintaining a abiding claret pH is critical to a person'southward well-being. The buffer that maintains the pH of homo blood involves carbonic acrid (HtwoCO3), bicarbonate ion (HCO3 –), and carbon dioxide (CO2). When bicarbonate ions combine with complimentary hydrogen ions and become carbonic acid, hydrogen ions are removed, moderating pH changes. Similarly, excess carbonic acrid tin be converted into carbon dioxide gas and exhaled through the lungs; this prevents too many costless hydrogen ions from edifice upwards in the claret and dangerously reducing its pH; likewise, if besides much OH– is introduced into the arrangement, carbonic acid will combine with it to create bicarbonate, lowering the pH. Without this buffer system, the body'south pH would fluctuate enough to jeopardize survival.
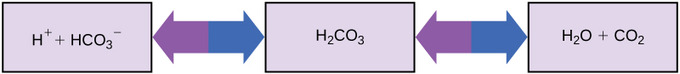
Buffers in the body: This diagram shows the body's buffering of blood pH levels: the bluish arrows show the process of raising pH as more CO2 is made; the purple arrows indicate the opposite procedure, lowering pH every bit more bicarbonate is created.
Antacids, which gainsay excess tummy acid, are another example of buffers. Many over-the-counter medications work similarly to claret buffers, often with at least one ion (commonly carbonate) capable of absorbing hydrogen and moderating pH, bringing relief to those that endure "heartburn" from stomach acid after eating.
Brønsted Acids and Bases
A Brønsted acid is any species capable of donating a proton; a Brønsted base is any capable of accepting a proton.
Learning Objectives
Identify the Brønsted acid, Brønsted base, conjugate acid, and conjugate base in an acid-base of operations reaction.
Key Takeaways
Key Points
- The Brønsted-Lowry theory is defined past the post-obit reaction: acid + base <=> conjugate base + conjugate acid. A conjugate base forms after the acid loses a proton, while the conjugate acid forms when the base accepts the proton. The reaction tin can keep in either management.
- The Brønsted-Lowry acid-base theory has several advantages over the Arrhenius theory: for example, only the Brønsted theory describes the reaction between acetic acrid and ammonia, which does not produce hydrogen ions in solution.
- H2o is amphoteric, which means information technology can act as either an acid or a base.
Key Terms
- Brønsted-Lowry base: any chemical species that acts equally an acceptor of protons
- Brønsted-Lowry acid: any chemic species that acts as a donor of protons
- cohabit base: the species formed afterwards an acid donates its proton; typically a weak base of operations
- cohabit acid: the species formed later a base accepts a proton; typically a weak acid
In chemical science, the Brønsted-Lowry theory is an acid-base of operations theory, independently proposed past Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. In this system, acids and bases are divers as follows: an acid is any species that is able to donate a hydrogen cation (H+, a proton); a base is whatsoever species with the ability to accept a hydrogen cation (H+). To that end, if a compound is to behave as an acid by altruistic a proton, at that place must be a base to accept that proton; the Brønsted-Lowry concept is therefore defined by the reaction:
acrid + base of operations ⇌ conjugate base + conjugate acid
The conjugate base of operations is the ion or molecule that remains after the acid has donated its proton, and the conjugate acid is the species created later the base of operations accepts the proton. The reaction can proceed either frontward astern; in each case, the acid donates a proton to the base of operations.
Advantages of Brønsted-Lowry Theory
The Brønsted-Lowry acid-base theory has several advantages over the Arrhenius theory. Retrieve that the Arrhenius theory defines an acid as whatsoever species that increases the concentration of H+/HthreeO+ in solution. Consider the post-obit reactions of acetic acid (CH3COOH), the organic acid that gives vinegar its feature taste:
- CHiiiCOOH + H2O ⇌ CHthreeCOO– + HiiiO+
- CH3COOH + NH3 ⇌ CH3COO– + NH4 +
Both theories hands depict the first reaction: CH3COOH acts every bit an Arrhenius acid because it acts equally a source of H3O+ when dissolved in water, and it acts as a Brønsted acid by altruistic a proton to water. In the second instance CHthreeCOOH undergoes the aforementioned transformation, in this case donating a proton to ammonia (NH3); this cannot be described using the Arrhenius definition of an acid, still, because the reaction does non produce HthreeO+.
Amphoterism of Water
Water is amphoteric, which means it can human activity every bit either an acid or a base. In the reaction between acerb acid, CHiiiCO2H, and water, H2O, water acts every bit a base. The acetate ion CHthreeCO2 – is the conjugate base of acetic acid, and the hydronium ion H3O+ is the conjugate acid of the base, water:
CH3COOH + H2O ⇌ CHthreeCOO– + H3O+
Water can also act as an acid, as when it reacts with ammonia. The equation given for this reaction is:
HtwoO + NH3 ⇌ OH– + NH4 +
Here, H2O donates a proton to NH3. The hydroxide ion is the conjugate base of water, which acts as an acid, and the ammonium ion is the conjugate acid of the base, ammonia.
Acid-Base of operations Titrations
Acrid-base of operations titration can determine the concentrations of unknown acrid or base solutions.
Learning Objectives
Compute the concentration of an unknown acrid or base given its volume and the volume and concentration of the standardized titrant.
Key Takeaways
Cardinal Points
- An acid – base titration is a quantitative assay of acids and bases; through this process, an acid or base of known concentration neutralizes an acrid or base of operations of unknown concentration.
- The titration progress tin can be monitored by visual indicators, pH electrodes, or both.
- The reaction's equivalence point is the point at which the titrant has exactly neutralized the acid or base in the unknown analyte; if you know the volume and concentration of the titrant at the equivalence betoken, you tin calculate the concentration of a base or acid in the unknown solution.
Key Terms
- analyte: the unknown solution whose concentration is being determined in the titration
- equivalence signal: the signal at which an added titrant's moles are stoichiometrically equal to the moles of acrid/base of operations in the sample; the smallest corporeality of titrant needed to fully neutralize or react with the analyte
- acid-base of operations titration: determines the concentration of an acid or base by exactly neutralizing it with an acid or base of known concentration
- titrant: the standardized (known) solution (either an acid or a base of operations) that is added during titration
Setting upwards an Acid-Base Titration
An acid-base titration is an experimental procedure used to determined the unknown concentration of an acid or base of operations by precisely neutralizing it with an acrid or base of known concentration. This lets us quantitatively clarify the concentration of the unknown solution. Acid-base titrations tin besides exist used to quantify the purity of chemicals.
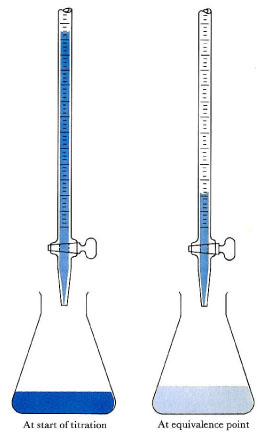
Acrid-base titration: The solution in the flask contains an unknown number of equivalents of base (or acrid). The burette is calibrated to show book to the nearest 0.001 cm3. It is filled with a solution of stiff acid (or base) of known concentration. Small increments are added from the burette until, at the terminate betoken, 1 drop changes the indicator color permanently. (An indication of the approaching equivalence point is that the indicator changes colour just changes back later on stirring.) At the equivalence point, the total amount of acid (or base) is recorded from the burette readings. The number of equivalents of acid and base must be equal at the equivalence point.
Alkalimetry, or alkimetry, is the specialized analytic employ of acid-base titration to decide the concentration of a basic (alkaline) substance; acidimetry, or acidometry, is the same concept applied to an acidic substance.
Materials for a Titration Procedure
- burette
- white tile (used to encounter a colour change in the solution)
- pipette
- pH indicator (the type depends on the reactants )
- Erlenmeyer or conical flask
- titrant (a standard solution of known concentration; a common example is aqueous sodium carbonate)
- analyte, or titrand (the solution of unknown concentration)
Equivalence Point Indicators

Acid-base titration setup: The pink colour is acquired by the phenolphthalein indicator.
Before you begin the titration, you must choose a suitable pH indicator, preferably one that volition experience a color change (known as the "end point") close to the reaction's equivalence point; this is the point at which equivalent amounts of the reactants and products accept reacted. Below are some common equivalence point indicators:
- strong acrid – strong base titration: phenolphthalein indicator
- weak acid – weak base titration: bromthymol blueish indicator
- strong acid-weak base titration: methyl orangish indicator the base is off the calibration (e.thou., pH > 13.5) and the acid has pH > 5.5: alizarine yellow indicator
- the base of operations is off the scale (eastward.thousand., pH > 13.5) and the acrid has pH > v.v: alizarine xanthous indicator
- the base is off the calibration (due east.g., pH > 13.five) and the acid has pH > v.5: alizarine yellow indicator
- the acrid is off the scale (e.one thousand., pH < 0.5) and the base has pH < eight.5: thymol blue indicator
Estimating the Equivalence Point'south pH
The resulting solution at the equivalence point volition have a pH dependent on the acid and base of operations's relative strengths. Y'all tin estimate the equivalence point's pH using the following rules:
- A strong acid will react with a weak base of operations to course an acidic (pH < 7) solution.
- A strong acid will react with a strong base to form a neutral (pH = seven) solution.
- A weak acid will react with a strong base to grade a basic (pH > seven) solution.
When a weak acrid reacts with a weak base of operations, the equivalence point solution will be basic if the base is stronger and acidic if the acrid is stronger; if both are of equal strength, then the equivalence pH will be neutral. Weak acids are not oft titrated against weak bases, however, considering the colour change is brief and therefore very difficult to observe.
Yous can determine the pH of a weak acrid solution beingness titrated with a strong base solution at diverse points; these fall into four dissimilar categories: (1) initial pH; (2) pH before the equivalence bespeak; (three) pH at the equivalence betoken; and (4) pH after the equivalence point.

Titration of a weak acid by a strong base: The pH of a weak acid solution being titrated with a strong base solution can exist found at each indicated point.
Titration Procedure
- Rinse the burette with the standard solution, the pipette with the unknown solution, and the conical flask with distilled water.
- Identify an accurately measured volume of the analyte into the Erlenmeyer flask using the pipette, along with a few drops of indicator. Place the standardized solution into the burette, and signal its initial volume in a lab notebook. At this phase, we want a rough guess of the amount of known solution necessary to neutralize the unknown solution. Let the solution out of the burette until the indicator changes colour, and record the value on the burette. This is the first titration and it is not very precise; it should exist excluded from any calculations.
- Perform at to the lowest degree 3 more than titrations, this time more accurately, taking into account where the end indicate will roughly occur. Record the initial and last readings on the burette, prior to starting the titration and at the end point, respectively. (Subtracting the initial volume from the concluding volume will yield the amount of titrant used to attain the endpoint.)
- The end point is reached when the indicator permanently changes color.
Gas Evolution Reactions
A gas development reaction is a chemical process that produces a gas, such as oxygen or carbon dioxide.
Learning Objectives
Identify when a reaction will evolve a gas.
Central Takeaways
Key Points
- Acids react with carbonates to produce a salt, carbon dioxide, and water.
- Limewater tin exist used to detect the presence of carbon dioxide evolved in a reaction.
- Hydrogen gas and a metallic table salt are produced when acids react with metals.
Key Terms
- gas development reaction: a chemic process that produce a gas, such every bit oxygen or carbon dioxide
- limewater: an aqueous solution of Ca(OH)2; a mutual indicator used to detect the presence of carbon dioxide gas
A gas development reaction is a chemic procedure that produces a gas, such equally oxygen or carbon dioxide. In the following examples, an acrid reacts with a carbonate, producing salt, carbon dioxide, and water, respectively.
Nitric acrid reacts with sodium carbonate to grade sodium nitrate, carbon dioxide, and water:
[latex]2\text{HNO}_{iii}(aq)+\text{Na}_2\text{CO}_{3}(aq)\rightarrow2\text{NaNO}_{3}(aq)+\text{CO}_{2}(g)+\text{H}_{2}\text{O}(l)[/latex]
Sulfuric acid reacts with calcium carbonate to form calcium sulfate, carbon dioxide, and water:
[latex]\text{H}_{2}\text{And so}_{4}(aq)+\text{CaCO}_{3}(aq)\rightarrow\text{CaSO}_{4}(aq)+\text{CO}_{2}(g)+\text{H}_{two}\text{O}(l)[/latex]
Muriatic acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water:
[latex]2\text{HCl}(aq)+\text{CaCO}_{3}(aq)\rightarrow\text{CaCl}_{2}(aq)+\text{CO}_{ii}(g)+\text{H}_{2}\text{O}(50)[/latex]
The following setup demonstrates this type of reaction:

Reaction of acids with carbonates: In this reaction setup, lime h2o is poured into ane of the test tubes and sealed with a stopper. A small-scale corporeality of muriatic acid is carefully poured into the remaining test tube. A small amount of sodium carbonate is added to the acid, and the tube is sealed with a safety stopper. The two tubes are connected. Equally a consequence of the acid-carbonate reaction, carbon dioxide is produced and the lime water turns milky.
The test tube on the correct contains limewater (a solution of calcium hydroxide, Ca(OH)2). On the left, a solution of muriatic acid has been added to a solution of sodium carbonate to generate [latex]\text{CO}_2(g)[/latex]. The exam tubes are sealed with rubber stoppers and continued with a delivery tube. As the reaction proceeds, the limewater on the correct turns from clear to milky; this is due to the [latex]\text{CO}_2(g)[/latex] reacting with the aqueous calcium hydroxide to form calcium carbonate, which is simply slightly soluble in water. The entire experiment is illustrated in the following video:
When this experiment is repeated with nitric or sulfuric acid instead of HCl, it yields the same results: the clear limewater turns milky, indicating the production of carbon dioxide.
Oxidation of Metals in Acidic Solution
The oxidation of a metal in acidic solution volition yield a metal common salt and hydrogen gas:
[latex]2\text{HCl}_{\left(aq\right)}+\text{Zn}_{\left(s\right)}\rightarrow\text{ZnCl}_{ii\left(aq\right)}+\text{H}_{2\left(g\right)}[/latex]
Acid and metal reaction: Hydrochloric acid oxidizes zinc to produce an aqueous metal salt and hydrogen gas bubbles.
Retrieve that oxidation refers to a loss of electrons, and reduction refers to the gain of electrons. In the higher up redox reaction, neutral zinc is oxidized to Zn2+, and the acid, H+, is reduced to H2(g). The oxidation of metals by strong acids is another common instance of a gas evolution reaction.
Source: https://courses.lumenlearning.com/boundless-chemistry/chapter/acid-base-reactions/
Posted by: pilgrimanable.blogspot.com


0 Response to "What Chemicals Help Reduce A Change In Ph When Acids Are Added To A Solution?"
Post a Comment